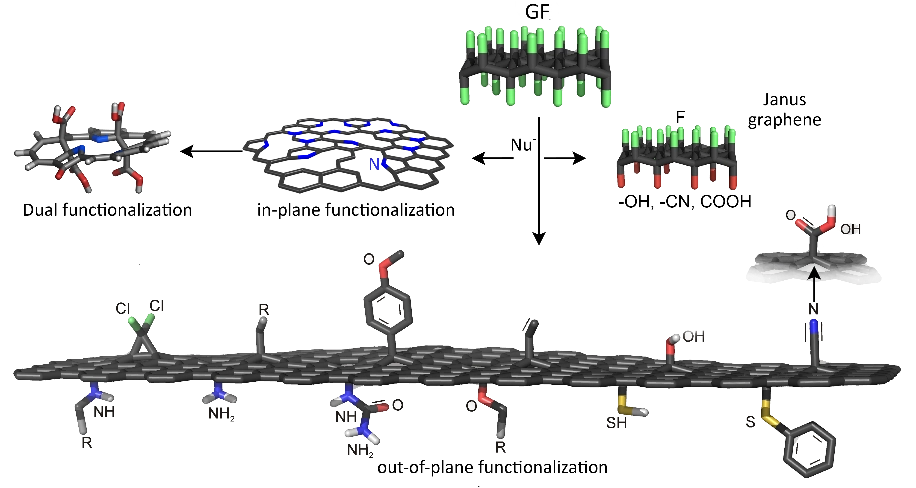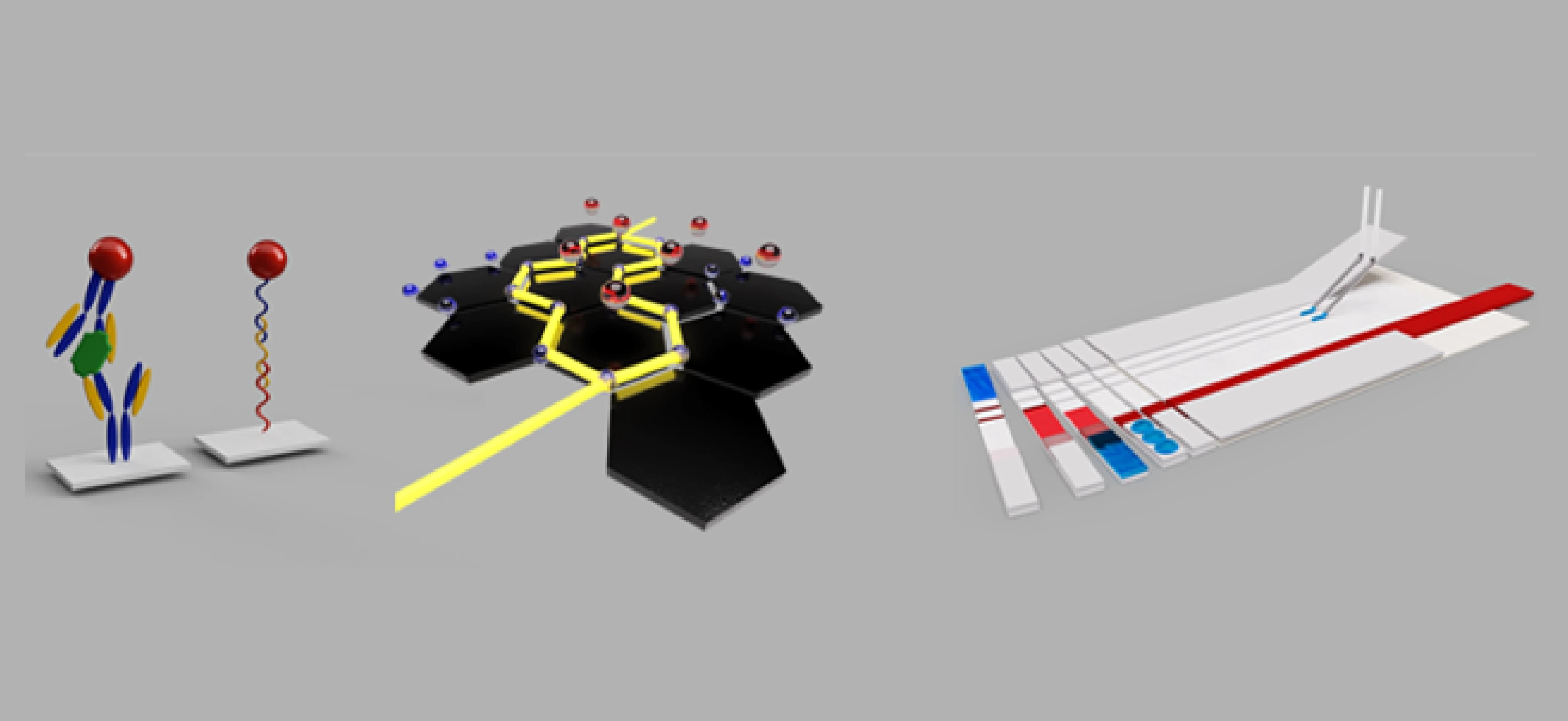
ICN2 provides a wide range of advanced technologies for electrochemical biosensing. Our expertise includes precise immobilization of bioreceptors, advanced functionalization of nanomaterials, state-of-the-art inkjet printing, and innovative graphene laser scribing techniques. These technologies work together to create highly efficient biosensing devices capable of both electrochemical and optical readouts. We specialise in adapting these methodologies to various substrates, such as paper and PET, to provide versatility in application. Our integrated approach ensures that our offerings deliver robust performance, heightened sensitivity, and specificity, addressing various environmental and analytical challenges. Our technology portfolio empowers the development of cutting-edge biosensing solutions, whether in bioreceptor engineering, nanomaterial modification, or substrate compatibility.