2D-BioPAD goes live at the center of the biomarker vs. neuropsychology debate
Our partner Alzheimer Hellas had the opportunity to present 2D-BioPAD at the hybrid scientific workshop “Controversies in Neuropsychological...
Read more
2D-BioPAD is pioneering a graphene-based Point-of-Care (PoC) In-Vitro Diagnostics (IVD) system for early Alzheimer’s Disease (AD) detection. This non-invasive solution, using 2D materials, can identify and quantify up to 5 AD biomarkers in real-time. Clinical pilot studies in Finland, Greece, and Germany aim to demonstrate its impact, with a focus on safety, ethics, and seamless integration into primary healthcare settings.


Our partner Alzheimer Hellas had the opportunity to present 2D-BioPAD at the hybrid scientific workshop “Controversies in Neuropsychological...
Read more

On January 24, 2026, Magda Tsolaki from Alzheimer Hellas gave an in-person lecture in the Master’s Program “Neurosciences...
Read more
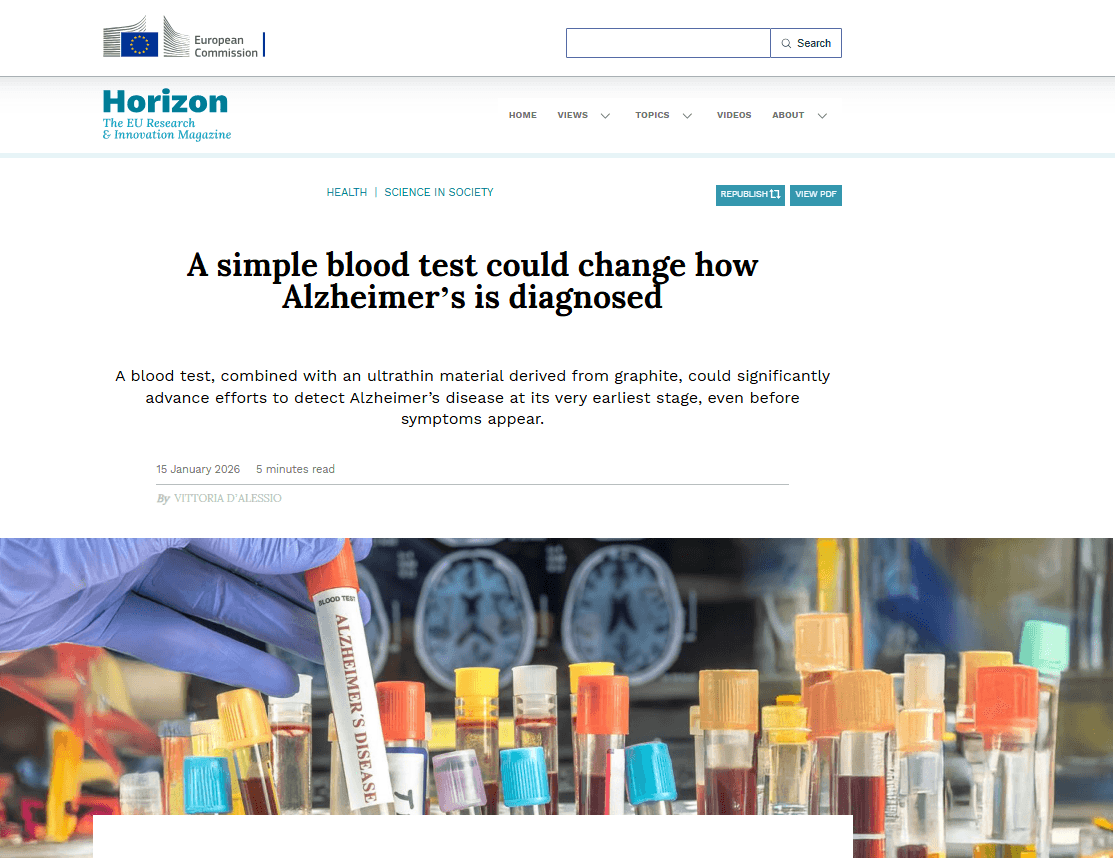
The 2D-BioPAD project was featured in Horizon, the EU Research and Innovation Magazine, in an article titled “A...
Read more

Our partner NOVAPTECH participated in BioFIT 2025, held in Strasbourg, one of Europe’s leading events dedicated to life-science...
Read more