
Materials in the nanoscale have unique properties that set them apart from regular materials, making them ideal for several biomedical applications. Out of a large pool of nanomaterials, Magnetic Nanoparticles (MNPs) are one of the most widely researched and applied nanomaterials in life sciences.
MNPs offer a range of unique qualities that make them crucial for many uses. They have a high surface-to-volume ratio, a superparamagnetic nature, high biocompatibility, low toxicity, and site-specific targeting. On top of these benefits, their cost-effective and sustainable manufacturing makes them great materials for various biomedical applications[1].
When combined with biosensors or aptasensors, MNPs can enhance the overall biosensing sensitivity, specificity and reliability[2]. MNPs help sample purification and avoid non-specific signals. They also play a crucial role in controlling the flow at different stages of the bioassay e.g., incubation of bioreceptors with the sample, purification, recognition, and signal acquisition[3],[4].
Behind the Curtain: How it works
Magnetically responsive nanoparticles can be designed to seek out, connect and interact with specific proteins. They can do this either directly (via appropriate surface functionalisation) or indirectly (via conjugation with probes such as antibodies or aptamers). With the help of an external magnetic field, we can control these tiny particles with high precision.
This magnetic interaction can help us measure the quantity of the bound analyte (e.g., a protein) in two ways: either through supporting fine-tuned separation (leaving only the target analyte in a known buffer) or by reading out magnetic signals (i.e., magnetic resistance, magnetic induction, nonlinear magnetization, etc.). Since there is little magnetic background signal from biological samples, this approach minimizes noise and interference[5].
The combination of these attributes can be valuable tools for bioassays, especially for Point-of-Care (PoC) IVDs[6].
By carefully choosing the strength and direction of (field intensity and field gradient) the external magnetic field, MNP and aptamer pairs/conjugates can be retained in specific areas. The magnetic field is then directed towards the electrodes where detection (recognition process) occurs. Any MNPs not binding to the electrode are eliminated towards an absorbent pad. At each step, the magnetic field parameters (field amplitude, duration, field sign) will be independently optimized to maximize the signal acquisition.
Are MNPs the solution for Alzheimer’s Disease biosensing?
Up to date a variety of nanoparticles (NPs) have been employed in biosensing techniques for AD, including gold and silver NPs, quantum dots, graphene oxide NPs, Prussian Blue NPs, carbon nanostructures, and various forms of MNPs[7],[8].
Their unique properties have been used to identify blood biomarkers, such as Aβ40, Aβ42 and p-tau[9],[10], with good selectivity and specificity, fast response and low level of detection (LOD), with several applications across the AD continuum.
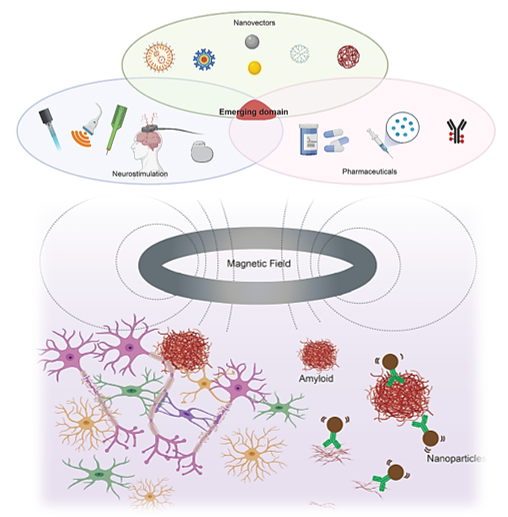
Figure 1 Top: Neurotechnologies based on magnetic fields and magnetic nanoparticles for AD. Bottom: Potential clinical integration can include nanoparticles as both a diagnostic tool and potential therapeutic modality.7
MNPs for Graphene-based biosensors in 2D-BioPAD
In 2D-BioPAD, the MagnaCharta Group (Magnetic Nanostructure Characterization: Technology and Applications) of the School of Physics at the Aristotle University of Thessaloniki (AUTh) synthesises tailor made MNPs to conjugate with aptamers and antibodies.
Magnetite (Fe3O4) MNPs have been synthesised while electrochemical sensing is required. To make them electrically conductive, they are transformed into hybrid particles in a second step. These Janus MNPs have a gold contact on one side and a magnetic part on the other.
The role of the magnetic counterpart is to enhance sensitivity and reliability via sample purification, minimalization of non-specific signals, and flow regulation during various stages of bioassays, such as bioreceptor incubation, purification, recognition, and signal acquisition.
The Au (gold) component acts as an electrochemical signal enhancer. It’s already proven effective in detecting key blood biomarkers like Aβ40, Aβ42, and p-Tau, showing good selectivity, specificity, and fast response. Plus, it has a low LOD, across different stages of AD.
To date, AUTh has successfully synthesized and structurally characterized several biphasic magnetite/gold nanoparticles (Fe₃O₄/Au), with variable size composition and morphology distinct NP systems, depicted in Figure 2. Synthesized NPs were characterized and adequately functionalized before and after the conjugation with DNA.
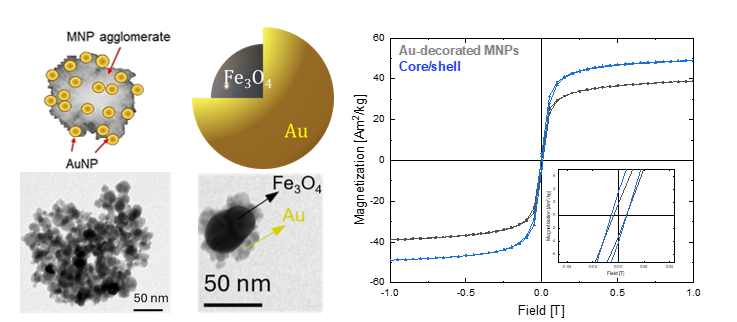
Figure 2 Nanoparticle formulations composed of Au and Fe3O4 (Au-decorated MNPs and Core/Shell), corresponding TEM images and Room-temperature hysteresis loops.
Stay tuned to learn more about these fascinating applications of graphene for cutting-edge biosensing solutions.
[1] Chavan, N., Dharmaraj, D., Sarap, S., & Surve, C. (2022). Magnetic nanoparticles–A new era in nanotechnology. Journal of Drug Delivery Science and Technology, 77, 103899.
[2] Le, T. D., Suttikhana, I., & Ashaolu, T. J. (2023). State of the art on the separation and purification of proteins by magnetic nanoparticles. Journal of Nanobiotechnology, 21(1), 363.
[3] Esmaeili, E., Ghiass, M. A., Vossoughi, M., & Soleimani, M. (2017). Hybrid Magnetic-DNA Directed Immobilisation Approach for Efficient Protein Capture and Detection on Microfluidic Platforms. Scientific reports, 7(1), 194.
[4] Zhu, N., Zhang, A., He, P., Fang, Y. (2004). DNA Hybridization at Magnetic Nanoparticles with Electrochemical Stripping Detection. Electroanalysis, 16(23), 1925-1930
[5] Cao, B., Wang, K., Xu, H., Qin, Q., Yang, J., Zheng, W., ... & Cui, D. (2020). Development of magnetic sensor technologies for point-of-care testing: Fundamentals, methodologies and applications. Sensors and Actuators A: Physical, 312, 112130.
[6] Hou, F., Sun, S., Abdullaha, S. W., Tang, Y., Li, X., & Guo, H. (2023). The application of nanoparticles in point-of-care testing (POCT) immunoassays. Analytical Methods.
[7] Ning S., Jorfi M., Patel S. R., Kim D.K., Tanzi, R.E. (2022) Neurotechnological Approaches to the Diagnosis and Treatment of Alzheimer’s Disease, Frontiers in Neuroscience, 16, 854992.
[8] Abdullah, S. A., Najm, L., Ladouceur, L., Ebrahimi, F., Shakeri, A., Al‐Jabouri, N., ... & Dellinger, K. (2023). Functional Nanomaterials for the Diagnosis of Alzheimer's Disease: Recent Progress and Future Perspectives. Advanced Functional Materials, 33(37), 2302673.
[9] Devi, R., Gogoi, S., Dutta, H. S., Bordoloi, M., Sanghi, S. K., & Khan, R. (2020). Au/NiFe 2 O 4 nanoparticle-decorated graphene oxide nanosheets for electrochemical immunosensing of amyloid beta peptide. Nanoscale Advances, 2(1), 239-248.
[10] Chiu, M. J., Chen, T. F., Hu, C. J., Yan, S. H., Sun, Y., Liu, B. H., ... & Yang, S. Y. (2020). Nanoparticle-based immunomagnetic assay of plasma biomarkers for differentiating dementia and prodromal states of Alzheimer's disease–A cross-validation study. Nanomedicine: Nanotechnology, Biology and Medicine, 28, 102182.