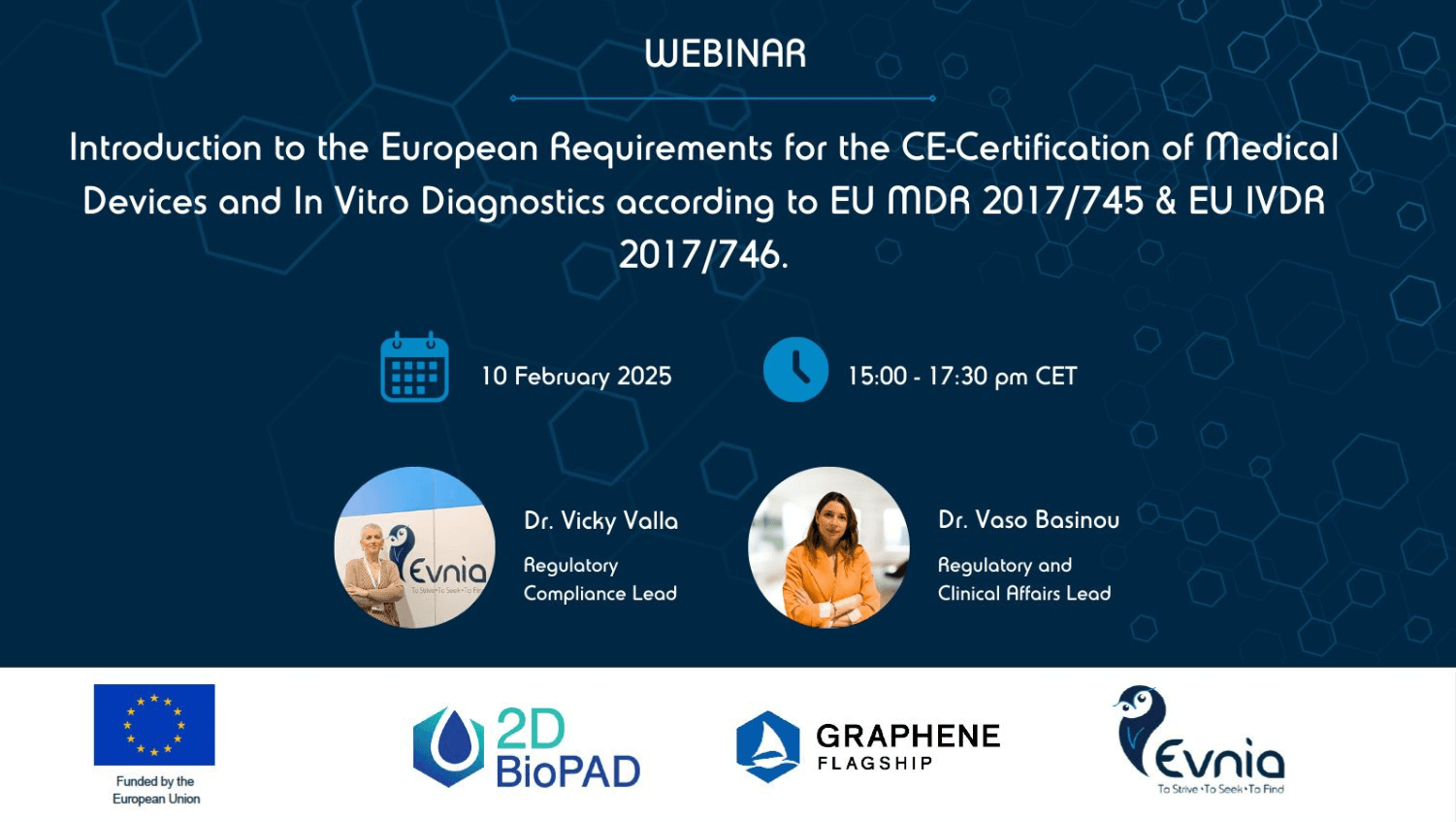
As a member of the 2D-BioPAD Consortium, Evnia hosts a webinar to discuss the basics of European Regulations for medical devices and in-vitro diagnostics.
When? 10 February 2025
Time? 15:00-17:30 pm CET
Join us in an open discussion for:
- MD/IVD development roadmaps
- the main challenges/bottlenecks associated with the current requirements
- the current status of the regulations' implementation and their impact on healthcare innovation
- an exchange of views on strategies for the lifecycle management of medical products
Secure your spot.