
Benefits and limitations
Paper-based lateral-flow biosensor assays (LFA) has become a very valuable and popular tool for Point-of-Care (PoC) In-Vitro Diagnostics (IVD)[1][2] proving to be excellent analytical tools for detecting a wide range of targets, including Alzheimer’s Disease (AD) biomarkers, due to their ease of use, portability, real-time analysis in a single-step[3] and, above all, cost-effective manufacturing.
However, LFAs face several limitations, such as:
- low sensitivity
- binary results (positive/negative),
- difficulty in handling complex matrices such as blood or serum,
- limited biomarker detection
These limitations hinder the adoption of such techniques for pathologies such as AD.
Electrochemical biosensors have been applied on several occasions in detecting biomarkers for neurodegenerative diseases, including AD. Most studies in recent literature focus on Αβ42, a central peptide in AD, primarily in lab set-ups with a limited number of samples.
The Rise of ePADs
Electrochemical paper-based analytical devices (ePADs), introduced by Dungchai et al. in 2009[4], has shown the rise of electrical readout devices such as glucometers or the most recent digital pregnancy tests. Those devices generate an electrochemical signal directly related to the amount of analyte (e.g., protein) present in the sample, further increasing the capability to act as a PoC sensor. In principle, ePADs are sensitive, portable, disposable and cost-effective (considerably due to the paper substrate) over conventional systems[5].
How it works
Electrochemical biosensors convert biochemical signals into electrical signals (current, potential, conductance, or impedance) through redox reactions. The signal is measured by the transducer, providing quantitative results.
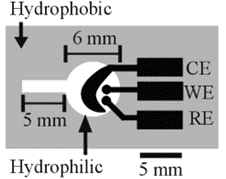
Figure 1. Basic design of the electrochemical detection cell for paper-based microfluidic devices. WE, working electrode; RE, reference electrode; CE, counter electrode8
2D-BioPAD Electrochemical Biosensors
A lot of research has followed the past decade, to optimise the functionality of such sensors, with emphasis on the technology of the electrodes. Under the Graphene Flagship a lot of significant progress has been made towards this direction, promising innovations that could soon bring more advanced biomedical technologies into clinical use.
In 2D-BioPAD, the partner Catalan Institute of Nanoscience and Nanotechnology (ICN2) brings a wide range of advanced technologies for electrochemical biosensing including precise immobilization of bioreceptors, advanced functionalization of nanomaterials, state-of-the-art inkjet printing, and innovative graphene laser scribing techniques. Their innovations yield highly efficient biosensors with digital electrochemical readouts for multiple analytes, including proteins.
ICN2 exploits patented technologies on print/stamp graphene-based electrode development on paper-like substrates[6], as well as cutting edge research[7], to achieve higher electrical signal and one-step functionalization compared to classic carbon screen-printed electrodes, to deliver robust performance, heightened sensitivity, and specificity, addressing various environmental and analytical challenges for multi analyte detection in blood samples.
Stay tuned for more!
[1] Parolo, C. et al., (2020). Tutorial: design and fabrication of nanoparticle-based lateral-flow immunoassays. Nature Protocols 15(12), 3788-3816.
[2] Sena-Torralba, A., et al. Toward Next Generation Lateral Flow Assays: Integration of Nanomaterials. Chemical Reviews, 122(18): 14881–14910, 2022
[3] Miku, E. (2021). Recent advancements in electrochemical biosensors for Alzheimer’s disease biomarkers detection. Current Medicinal Chemistry, 28(20), 4049-4073.
[4] Dungchai, W., Chailapakul, O., & Henry, C. S. (2009). Electrochemical detection for paper-based microfluidics. Analytical chemistry, 81(14), 5821-5826.
[5] Solhi, E., Hasanzadeh, M., & Babaie, P. (2020). Electrochemical paper-based analytical devices (ePADs) toward biosensing: recent advances and challenges in bioanalysis. Analytical methods, 12(11), 1398-1414.
[6] Giacomelli, C., et al. (2020). Selective stamping of laser scribed rGO nanofilms: From sensing to multiple applications. 2D Materials, 7(2), 024006.
[7] Calucho, E., et al. (2024). Reduced graphene oxide electrodes meet lateral flow assays: A promising path to advanced point-of-care diagnostics. Biosensors and Bioelectronics, 258, 116315.